Rivasterat(CU06) – DME, Wet-AMD
Therapeutic Funtion of Rivasterat in Diabetic Macular Edema
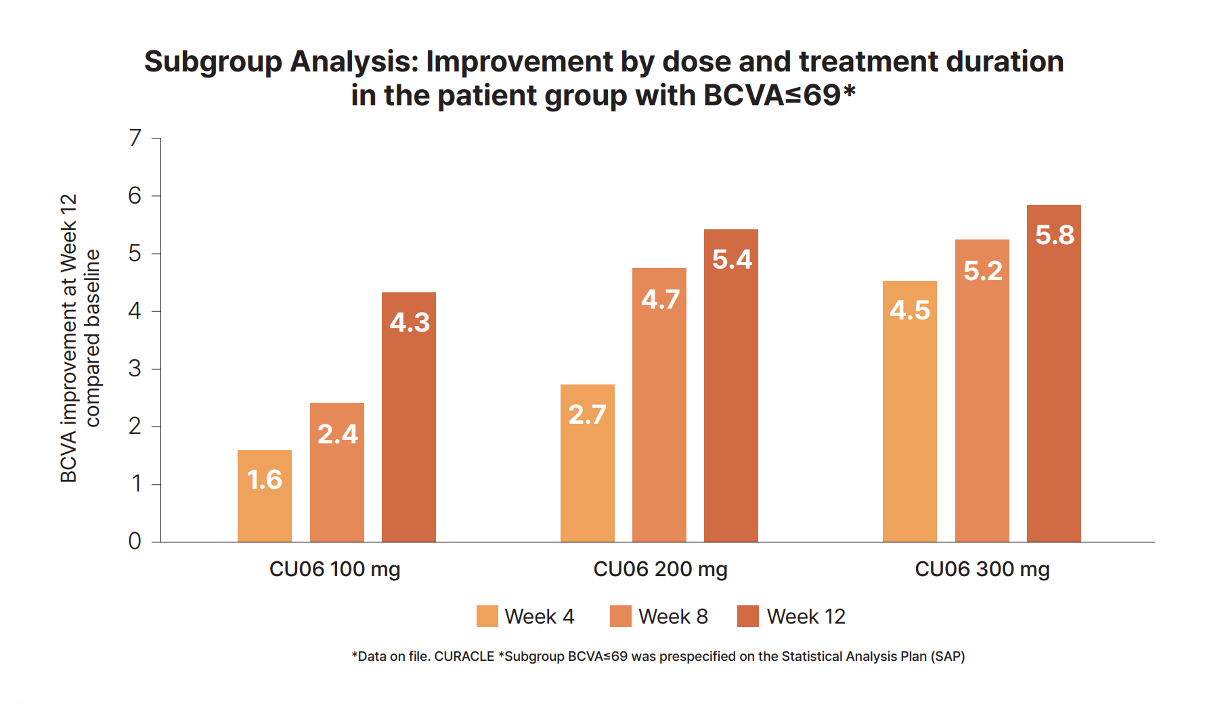
Ph2a results of Rivasterat
Dose- & treatment duration-dependent BCVA improvent in patients with poor vision
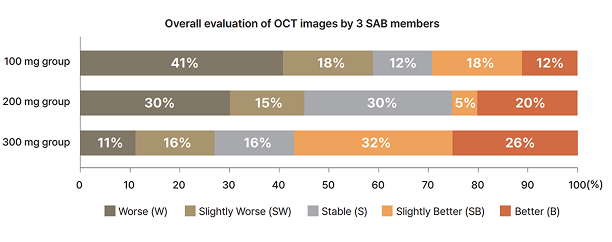
Ph2a results of Rivasterat
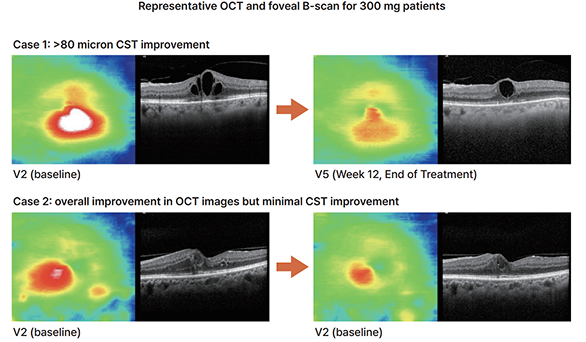
Dose- depentent anatomical improvement (OCT edema & structure) in Post Hoc Analysis
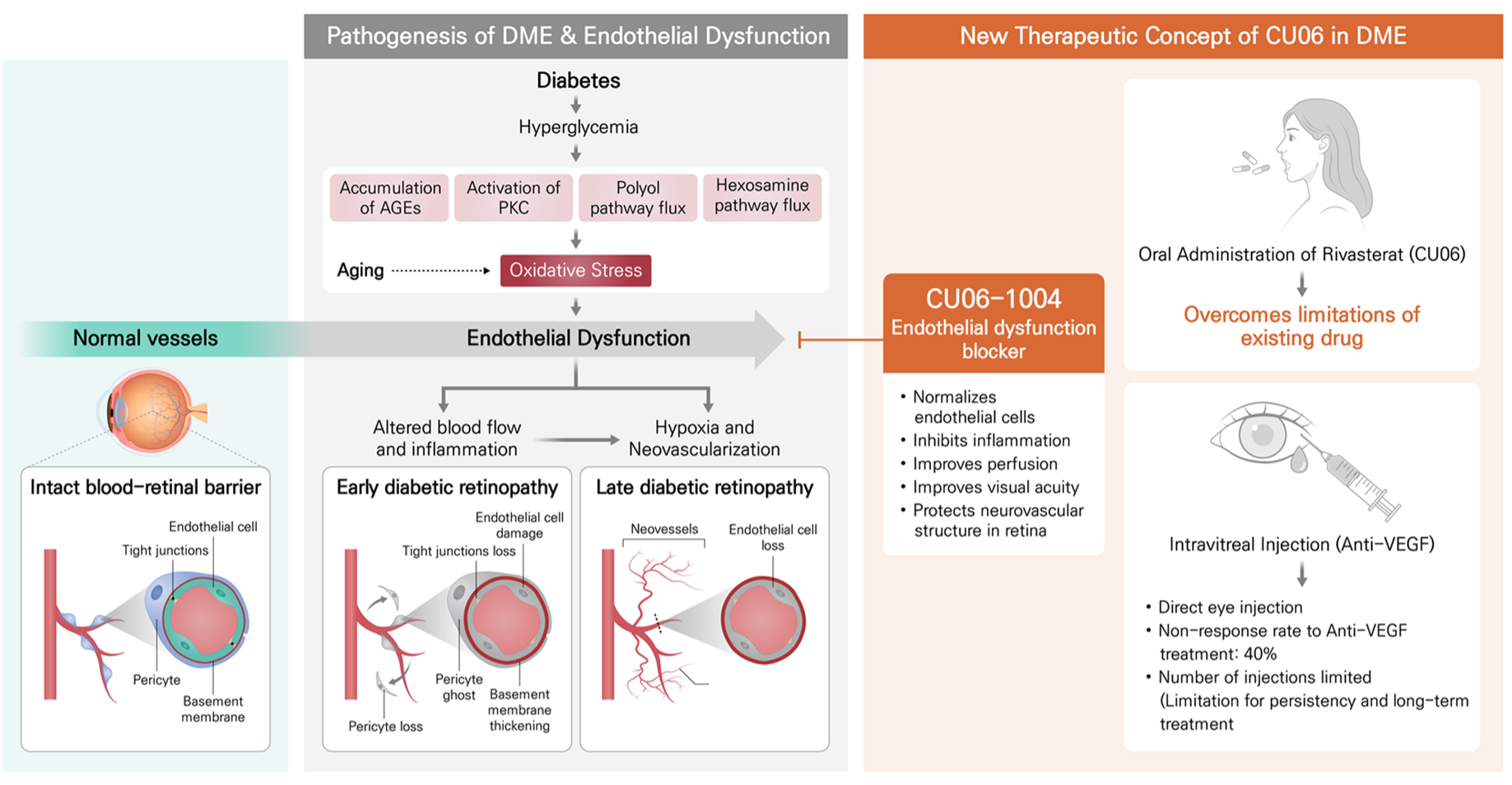
First in class Oral Endothelial Stabilizer
Differentiated endothelial raft modulator with a novel mechanism distinct from anti-VEGF and anti-Ang-2 injections.
Phase 2a (DME) Validation
Demonstrated BCVA gains, OCT-based vascular stabilization, excellent safety
Neurovascular Protection
Beyond edema control → endothelial protection & microvascular restoration, enabling neurovascular recovery
Unmet Needs
DME market projected to reach ~$7.5B by 2034, with >40% of patients partial/non-responders to anti-VEGF
Wet AMD market expected at $18–20B by 2030, burdened by frequent injections, limited adherence, and access issues
Therapeutic Value
With oral dosing convenience and a novel mechanism, Rivasterat is positioned for both stand-alone use and combination
with anti-VEGF, emerging as a next-generation treatment option