| Pipeline | Indication | Ad Route | Key Features | Development Status | Discovery |
Pre- Clinical |
Phase 1 | Phase 2 | Phase 3 | Launch | Partner | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IIa | IIb | |||||||||||
| Rivasterat (CU06) | DME(Diabetic Macular Edema) / Wet-AMD(Age-related Macular Degeneration) | PO | First oral endothelial dysfunction blocker | Completed Phase 2a clinical trial in the U.S(2024.1Q) | ||||||||
| CU01 | DN(Diabetic Nephropathy) | PO | Nrf2-activating, TGF-β-inhibiting therapy | Phase 2b clinical trial in Korea completed | ||||||||
| MT-101 | AKI(Acute Kidney Injury)/ CKD(Chronic Kidney Disease) | IV | Tie2-activating antibody | Preclinical studies in progress |

|
|||||||
| MT-103 | Wet-AMD / DME / DR(Diabetic Retinopathy) | IV | Tie2-activating x anti-VEGF(Bi-specific antibody) | Preclinical studies in progress |

|
|||||||
| MT-105 | IBD(Inflammatory Bowel Disease) | IV | trispecific antibody | Discovery |

|
|||||||
| MT-201 | DVT(Deep Vein Thrombosis) / PE(Pulmonary Embolism) | IV | Non bleeding anti-thrombotic antibody | Preclinical studies in progress |

|
|||||||
| MT-202 | AIS(Acute Ischemic Stroke) / HFpEF(Heart Failure with preserved Ejection Fraction) | IV | Non-bleeding anti-thrombotic × Tie2-activating (bispecific antibody) | Preclinical studies in progress |

|
|||||||
| CU71 | AD(Alzheimer’s Disease) | PO | Next-generation endothelial dysfunction blocker | Preclinical studies in progress | ||||||||
| CP01-R01 | Kidney Failure in Companion Animals | PO | Confirmed therapeutic effects on kidney disease in animal models | Phase 3 clinical trial in dogs ongoing (Korea) |

|
|||||||
| CU104 | UC(Ulcerative Colitis) | PO | Indication expansion of CU06 | Completed Phase 1 clinical trial in the U.S | ||||||||
| CU106 | Immuno-Oncology combination | PO | Indication expansion of CU06 | Completed Phase 1 clinical trial in the U.S | ||||||||
Where We Aim to Innovate
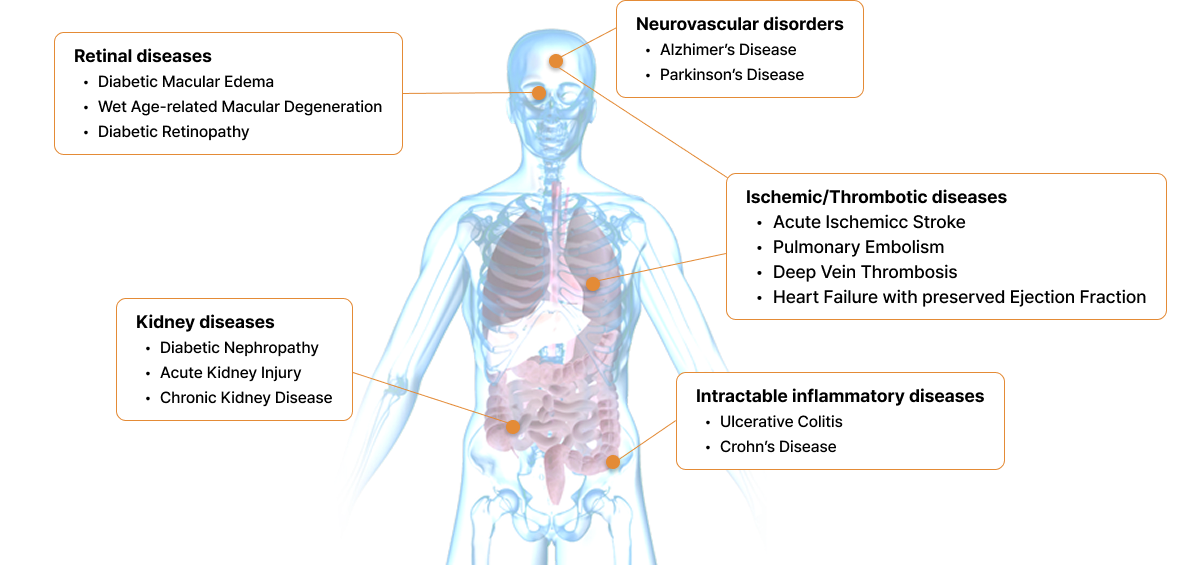
paradigms across the eye, kidney, brain, cardiovascular, and inflammatory diseases.
Rivasterat (CU06)
The first oral Endothelial Dysfunction Blocker.
Phase 2a in DME demonstrated vision improvement and safety, emerging as a next-generation oral therapy.
CU01
A dual-function therapy targeting NRF2 activation and TGF-β inhibition.
Phase 2 in diabetic nephropathy confirmed safety and GFR improvement, advancing as a next-generation renal therapy.
MT-101
The first fully humanized Tie2 agonist antibody.
Directly activates renal vasculature to reduce inflammation and fibrosis, emerging as an innovative therapy for AKI and CKD.
MT-103
The first bispecific antibody combining Tie2 agonist and anti-VEGF functions. Unlike Vabysmo, MT-103 directly activates Tie2 and has shown superior preclinical efficacy, defining a next-generation therapy for retinal diseases.
MT-105
Tie2-based trispecific antibody targeting IBD
MT-201
A non-bleeding anti-thrombotic antibody targeting PD-X.
Inhibits and dissolves clots in VTE and PE without bleeding risk, advancing as a next-generation anti-thrombotic therapy.
MT-202
A dual-mechanism therapy combining PD-X inhibition and Tie2 agonism.
Provides endothelial protection and anti-thrombotic efficacy in ischemia/reperfusion injury, developed as an innovative combined therapy.
CU71
Next-generation Neurovascular Stabilizer.
Targets BBB dysfunction and neuroinflammation, positioned as an innovative candidate for Alzheimer's, small vessel disease, and vascular dementia.
CP01-R01
First oral endothelial stabilizer–based candidate for companion animal CKD therapy.