MT-103 – DME, wAMD, DR
MT-103, Tie2 x VEGF bispecific Ab, for treating wAMD
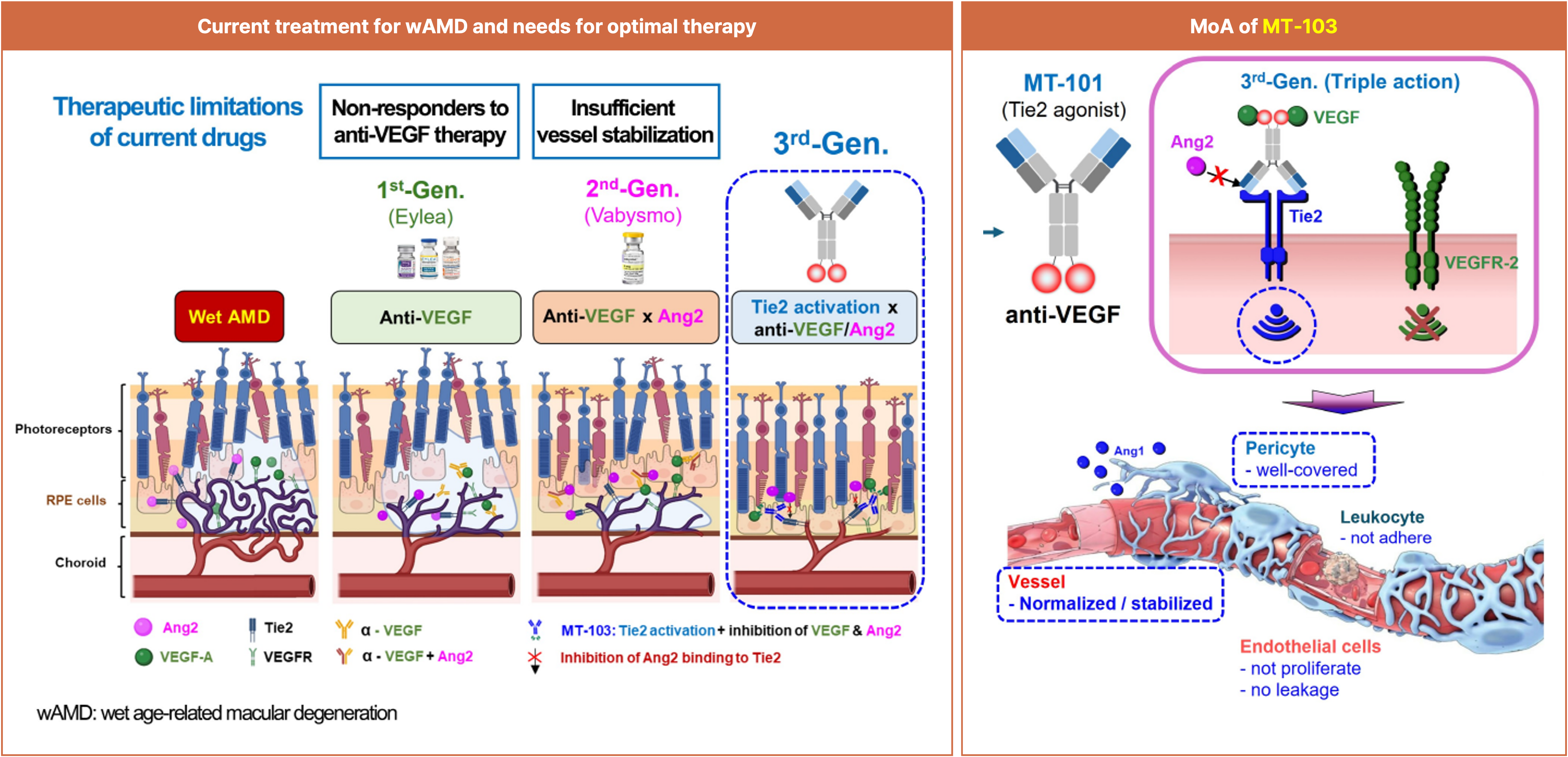
Next-generation bispecific antibody (Tie2 agonist + anti-VEGF)
Provides a triple mechanism with direct Tie2 activation plus VEGF and Ang-2 inhibition
Innovative mechanism
Beyond anti-angiogenesis, achieves vascular stabilization, permeability control, and anti-inflammation simultaneously
Preclinical efficacy
Demonstrated superior vascular stabilization and anti-angiogenic effects versus Vabysmo (faricimab) in head-to-head preclinical studies
Indications & unmet need
DME : ~7% of diabetic patients affected → ~21M patients by 2030
Wet AMD: ~20M patients worldwide, prevalence rising with aging populations
Despite advances, patients still face frequent injections and non-/partial response, creating demand for next-gen therapies
Commercial value
The global retinal disease market is projected to reach ~$20B by 2030.
MT-103 is positioned as a next-generation best-in-class candidate beyond Vabysmo, with strong scientific differentiation
through its triple mechanism and broad market potential